This review will highlight items to consider before you implement you clinical program to evaluate a new drug in this area, switch a prescription drug or extend the claims of an already existing drug.
Our staff has had more experience in this therapeutic area than virtually any other group designing the clinical programs, identifying the clinical centers, conducting the studies, analyzing the data and reporting these results both in final reports and in NDA submissions/publications.
SCOPE AND VARIABILITY OF EXPERTISE:
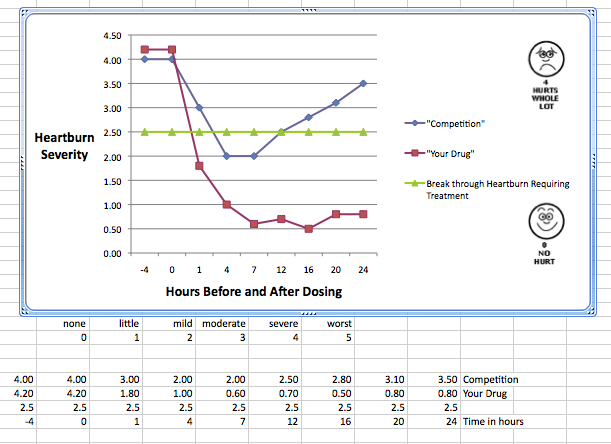
Through August 2013, we have designed and conducted heartburn studies evaluating over 24,800 subjects. It started, by us, long ago designing a ‘pizza’ study to assess metoclopramide’s effect to reduce heartburn because the drug sped motility. We worked our way through antacid claims with Tums and Rolaids Alka-Seltzer and Pepto-Bismol. Then bridging from duodenal and gastric ulcers effectiveness of the H2-Antagonists we successfully managed the pivotal studies for the leading drugs in this category. We worked with several cytoprotective agents and recently have conducted many studies with the PPIs.
The studies we have designed and conducted cover the full range of efficacy evaluations: single center methodology studies; pH capsule studies for pharmacodynamic/kinetic properties in normal as well as G.I. distressed subjects; through “at-Home”, “In-Use” studies and provocative meals. We have seen what works, what truly doesn’t work, how best to squeeze more efficacy out of a clinical program, and how to identify ‘weakness’ in a clinical that may be seeking too many goals and miss the chance to tighten the study design and thereby secure the primary goals.
If you are looking at your Rx drug and trying to determine if it should be switched OTC; possibly acquire an Rx drug to do the same, or combine it with other drugs to gain competitive advantage. Alternatively, if you have one of the existing drugs now on the market in this very active and competitive category, it is important to know these facts before you proceed down the road in securing new claims for your drug. You need to look 2 steps ahead to see what your drug will need to deliver in terms of safety and efficacy to remain a viable and hopefully the leading competitor in this marketplace.
Your product will need to make some, if not all, of the following claims:
“fastest time to relief”,
“complete relief in minutes”,
“less concomitant symptoms, no cramps, no gas…,”
‘best on the market – complete relief even after 2 provoking meals’
‘all day and all night complete relief’,
“easy to the g.i. system [as compared to others]”…
So what are the possibilities—let’s just picture one possible scenario in developing the next generation drug.
IHC’s expertise in this area can be instrumental in helping your company answer these important questions about your drug:
- What Claims can you make,
- With what Products to Test,
- With what clinical designs to Use and
- What population to test it against
Claims to Make
- Prevention of Heartburn – dosing prior to the heartburn episode
- Treatment of Heartburn – dosing from the first sign of heartburn
Products to Test
- Your Drug alone
- Your Drug in combination with one or several agents – antacids, H2Antaginists, cytoprotective agents…
Clinical Designs to Use
- pH Study to assess pH changes before, during and after meals
- Provocative Meal Studies – Crossover or Parallel
- At-Home Use Studies
Populations to Test
- Moderate Heartburn Sufferer – severity defined as : up to 3 weekly episodes due to food, stress, etc., as well as those failing to get relief some of the time from present H2Antagonists or PPI OTC products
- Severe Heartburn Sufferer – severity defined as those having almost daily episodes due to food, stress, etc., as well as those failing to get relief most of the time from present H2Antagonists or PPI OTC products
- The Range of Moderate to Severe – defining a category that includes the majority of frequent heartburn sufferers
Decisions have to be made on which way to go and especially taking into account the changing marketplace. You need to design your clinical program that
- Evaluates the potential of the next generation of drugs, including those that can be derived from existing products
- Delivers success initially by picking the Low Hanging Fruit – establish in quick clinical studies, your drugs superiority in treatment and/or prevention of the condition as compared to the older generations of drugs competing in this arena.
- Construct a Database of your present and future competition, via pilot studies, which will answer the important future questions:
- How does your drug compare to those drugs that are Rx?
- What about your drug vs. combos of several older generations of drugs?
- What about the ‘sister’ formulations of your drug?
- How does your drug compare to off-patent formulations of your drug in combo with older generation drugs?
- Develop this database of your competitors to be used to defend against their weak or unsubstantiated claims.
As the database grows in complexity and scope, a series of analytical tools can interrogate this database and show to marketing and clinical where, when, how and how much it will cost to make new claims, including new ground breaking competitive superiority claims…