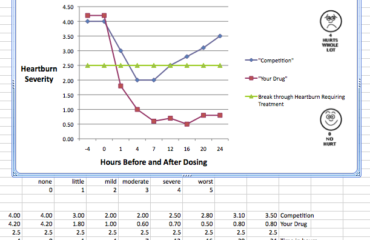
We are in the forefront of following the new Clinical Research Guidelines and trends from the FDA. Whether its structuring the clinical program to enable a risk-based approach to monitoring or designing the program to assess product efficacy (and safety) at prospective time points thereby adapting the study data collection plan to maximize the ‘interim’ results and significantly improve the value of the studies conducted.
Our role is to assist Sponsors in the design and implementation of their clinical program, to minimize outside factors so the efficacy and safety of their product rests on its laurels and not affected by outside conditions. To accomplish this we utilize our experience and expertise to assess whether a Risk-Based Approach to Monitoring will improve the quality of data and control costs. We also can provide an assessment of the study design to determine if an adaptive clinical design can improve the product chances for approval. When to interact with the FDA and to what extent should the study be ‘adaptive’ is a key aspect to achieving all the clinical benefits of adapting to such a design.
The risk-based approach focuses on critical study parameters and relies on a combination of monitoring activities to oversee a study effectively. For example, the guidance specifically encourages greater use of centralized monitoring methods where appropriate. This includes the utilization of centralized monitoring methods within our EDC (electronic data capture) and study metric workbook capabilities. We interrogate ongoing study data to empower our monitors to utilize all clinical site data available to prevent or mitigate risks to data quality while assuring protection of all subjects participating in the study. We act quickly to retrain clinical site staff including the Principal Investigators thereby keeping everyone abreast of critical study processes and endpoints.
Recent FDA draft Guidelines have laid out a framework of designing clinical trials that can be adaptive with features (such as – changes in design or analyses guided by examination of the accumulated data at an interim point in the trial) which would make studies more efficient (that is – shorter duration, fewer patients), more likely to demonstrate an effect of the drug if one exists, or more informative (e.g., by providing broader dose-response information)
This adaptive clinical study design approach provides sponsors with the opportunity to prospectively plan for a modification of one or more specified aspects of the study design and hypotheses based on analysis of data (usually interim data) from subjects in the study. Such analyses of the accumulated study data would be performed at prospectively planned time points within the study. This analysis can be performed in a fully blinded manner or in an unblinded manner, and can occur with or without formal statistical hypothesis testing.