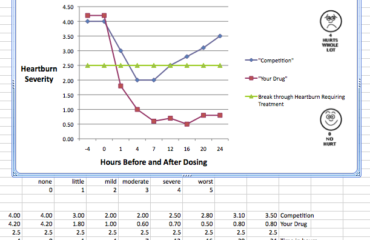
This review will highlight IHC’s expertise as you consider extending the claims of your drugs used in treating these and related clinical conditions. Our staff has had direct experience designing, conducting and analyzing clinical studies evaluating these drugs and other drugs that are in competition with you.
| Condition or Disease | Clinical Experience with Drugs used in the treatment of diarrhea, constipation and related disorders | |
| Diarrhea | Imodium® Lomotil® Pepto-Bismol® |
Donnagel® Kaopectate® Lotronex® |
| Constipation | MiraLAX® Citrucel® Dulcolax® |
Metamucil® Senokot® FiberCon® |
The scope of experience, expertise and creativity of the staff at IHC can be exemplified by the work we provided during the research, development and marketing of the above drugs.
One such expertise that truly exemplifies the unique and broad service we provide is evident in our management of the Imodium® brand –
Our management was asked by one of our client’s to help them switch Loperamide from Rx>OTC for acute non-specific diarrhea. When they came to us for assistance, the status of their program to that date was 53 enrolled patients in 40 centers throughout the US over a 2-year period. Their goal of 1500 patients was needed before submitting the results to the FDA. At the very least they were frustrated and considered foregoing the switch.
We redesigned their program and scoped out potential high volume sites and medical clinics we work with throughout Latin America. As a result, 6 clinical studies were initiated, completed and reported to the FDA in less than 15 months. A total of 5250 patients successfully completed these studies. This effort and quality data results enabled a successful filing with the FDA. The drug was approved for OTC use in patients with acute non-specific diarrhea.
Additionally these clinical results also provided the necessary evidence establishing the safety and efficacy of Imodium® for children > 6 yrs of age. Plus, the results also established the benefit of Imodium® in a geriatric population. Heretofore other products used to treat diarrhea resulted in adverse effects that complicated the lives of the geriatric population. It was clearly shown that the geriatric population tolerated their product very well.
IHC’s further creative and research orientated staff was responsible for the expansion of the Imodium® line of products. By observing the clinical diaries and concomitant medications taken by the some of the study participants, our staff observed a higher degree of overall relief when the study participants happen to also take a chewable tablet of Simethicone to relieve their concomitant gas they had with the diarrhea. We presented an ad hoc analysis of these subjects to the client and recommended that they consider pursuing this as a combo product extending their Imodium product line. The company did agree, we conducted the studies and the rest is history – the combo product was approved for marketing and is a big seller for the treatment of diarrhea.
Through our experience and expertise in managing large clinical programs for these drugs and other drugs, you can be assured that we will complete your clinical studies on-time and within budget.
SCOPE AND VARIABILITY OF EXPERTISE:
Through August, 2015, our staff has designed and conducted a wide range of studies evaluating over 7,500 subjects with diarrhea and/or constipation.
The studies conducted cover the full range of efficacy evaluations: single center methodology studies; studies assessing the pharmacodynamic/kinetic properties in normal as well as g.i. distressed subjects; through “at-Home”, generalized “Consumer-Use” studies and sequestered studies to evaluate overnight relief following a symptom provoking meal. We have seen what works, what truly doesn’t work, how best to squeeze more efficacy out of a clinical program, and how to identify ‘weakness’ in a clinical that may be seek too many goals and miss the chance to tighten the study design and thereby secure the primary goals.
Novartis is a major player in the gastrointestinal arena. As noted in the above table, we have highlighted some of the drugs we have tested for clients. It is evident that IHC has extensive experience evaluating drugs in this broad therapeutic area.
Whether your products are designed to mitigate the range of gastrointestinal symptoms, prevent them or cure them; IHC can provide strategic counseling, methodology development, proof of concept assessment through designing and implementing the full clinical program. With drugs like Benefiber®, the digestive aid Dycholium®, the overnight laxative Ex-LAX®, the mild heartburn episode reliever Maalox® or Perdiem®, or the excess gas reliever Gas-X®, we can assist Novartis in maximizing their claims. By understanding thoroughly the category we can provide invaluable assistance in protocol development as well as have the clinical centers that deliver the quality populations.