Diarrhea, Constipation: Treatment & Related Conditions
Friday, 19 February 2016
This review will highlight IHC’s expertise as you consider extending the claims of your drugs used in treating these and related clinical conditions. Our staff has had direct experience designing, conducting and analyzing clinical studies evaluating these drugs and other drugs that are in competition with you. Condition or Disease Clinical Experience with Drugs used
- Published in History of Expertise
No Comments
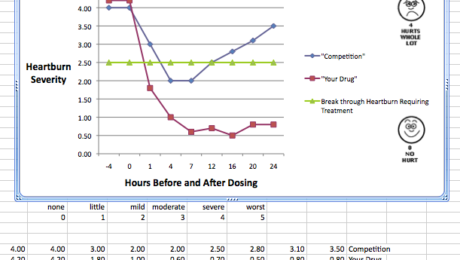
Heartburn Treatment & Prevention
Friday, 19 February 2016
This review will highlight items to consider before you implement you clinical program to evaluate a new drug in this area, switch a prescription drug or extend the claims of an already existing drug. Our staff has had more experience in this therapeutic area than virtually any other group designing the clinical programs, identifying the
- Published in History of Expertise
Clinical Expertise with Cardiovascular Drugs
Thursday, 20 February 2014
… And The Disorders They Treat IHC has had extensive experience and expertise designing, conducting and analyzing drugs used in the treatment of various cardiovascular disorders. We have presented below some details of the studies we conducted and managed with the goal to show the complexity of these programs and through the success of these
- Published in History of Expertise
Risk-Based Approach to Monitoring & Adaptive Clinical Research
Friday, 14 February 2014
We are in the forefront of following the new Clinical Research Guidelines and trends from the FDA. Whether its structuring the clinical program to enable a risk-based approach to monitoring or designing the program to assess product efficacy (and safety) at prospective time points thereby adapting the study data collection plan to maximize the ‘interim’
- Published in History of Expertise